This article is about acids in chemistry
An acid (from the Latin acidus/acēre meaning sour) is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH under 7, with acidity increasing the lower the pH. Chemicals or substances having the property of an acid are said to be acidic.
Common examples of acids include acetic acid ( CH3COOH )(in vinegar), sulfuric acid ( H2SO4(used in car batteries), and tartaric acid/racemic acid( C4H6O6 ) (used in baking). As these three examples show, acids can be solutions, liquids, or solids. Gases such as hydrogen chloride can be acids as well when dissolved in water. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
There are three common definitions for acids:
The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. Most acids encountered in everyday life are aqueous solutions, or can be dissolved in water, and these two definitions are most relevant. The reason why pHs of acids are less than 7 is that the concentration of hydronium ions is greater than 10−7 moles per liter. Since pH is defined as the negative logarithm of the concentration of hydronium ions, acids thus have pHs of less than 7. By the Brønsted-Lowry definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments.
In chemistry, the Lewis definition of acidity is frequently encountered. Lewis acids are electron-pair acceptors. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride. Hydronium ions are acids according to all three definitions. Interestingly, although alcohols and amines can be Brønsted-Lowry acids as mentioned above, they can also function as Lewis bases due to the lone pairs of electrons on their oxygen and nitrogen atoms.
The Swedish chemist Svante Arrhenius attributed the properties of acidity to hydrogen in 1884. An Arrhenius acid is a substance that increases the concentration of the hydronium ion, H3O+, when dissolved in water. This definition stems from the equilibrium dissociation of water into hydronium and hydroxide (OH−) ions:
H2O(l) + H2O(l) is in equilibrium with H3O+(aq) + OH−(aq)
In pure water the majority of molecules exist as H2O, but a small number of molecules are constantly dissociating and re-associating. Pure water is neutral with respect to acidity or basicity because the concentration of hydroxide ions is always equal to the concentration of hydronium ions. An Arrhenius base is a molecule which increases the concentration of the hydroxide ion when dissolved in water. Note that chemists often write H+(aq) and refer to the hydrogen ion when describing acid-base reactions but the free hydrogen nucleus, a proton, does not exist alone in water, it exists as the hydronium ion, H3O+
While the Arrhenius concept is useful for describing many reactions, it is also quite limited in its scope. In 1923 chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently recognized that acid-base reactions involve the transfer of a proton. A Brønsted-Lowry acid (or simply Brønsted acid) is a species that donates a proton to a Brønsted-Lowry base. Brønsted-Lowry acid-base theory has several advantages over Arrhenius theory. Consider the following reactions of acetic acid ( CH3COOH ), the organic acid that gives vinegar its characteristic taste
Both theories easily describe the first reaction: CH3COOH acts as an Arrhenius acid because it acts as a source of H3O+ when dissolved in water, and it acts as a Brønsted acid by donating a proton to water. In the second example CH3COOH undergoes the same transformation, in this case donating a proton to ammonia (NH3), but cannot be described using the Arrhenius definition of an acid because the reaction does not produce hydronium. Brønsted-Lowry theory can also be used to describe molecular compounds, whereas Arrhenius acids must be ionic compounds. Hydrogen chloride (HCl) and ammonia combine under several different conditions to form ammonium chloride, NH4Cl. In aqueous solution HCl behaves as hydrochloric acid and exists as hydronium and chloride ions. The following reactions illustrate the limitations of Arrhenius's definition:
H3O+(aq) + Cl−(aq) + NH3 → Cl−(aq) + NH4+(aq)
HCl(benzene) + NH3(benzene) → NH4Cl(s)
HCl(g) + NH3(g) → NH4Cl(s)
As with the acetic acid reactions, both definitions work for the first example, where water is the solvent and hydronium ion is formed. The next two reactions do not involve the formation of ions but are still proton transfer reactions. In the second reaction hydrogen chloride and ammonia (dissolved in benzene) react to form solid ammonium chloride in a benzene solvent and in the third gaseous HCl and NH3 combine to form the solid.
A third concept was proposed in 1923 by Gilbert N. Lewis which includes reactions with acid-base characteristics that do not involve a proton transfer. A Lewis acid is a species that accepts a pair of electrons from another species; in other words, it is an electron pair acceptor. Brønsted acid-base reactions are proton transfer reactions while Lewis acid-base reactions are electron pair transfers. All Brønsted acids are also Lewis acids, but not all Lewis acids are Brønsted acids. Contrast the following reactions which could be described in terms of acid-base chemistry.
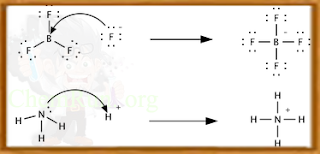
In the first reaction a fluoride ion, F−, gives up an electron pair to boron trifluoride (BF3to form the product tetrafluoroborate (BF4-. Fluoride "loses" a pair of valence electrons because the electrons shared in the B—F bond are located in the region of space between the two atomic nuclei and are therefore more distant from the fluoride nucleus than they are in the lone fluoride ion. BF3 is a Lewis acid because it accepts the electron pair from fluoride. This reaction cannot be described in terms of Brønsted theory because there is no proton transfer. The second reaction can be described using either theory. A proton is transferred from an unspecified Brønsted acid to ammonia, a Brønsted base; alternatively, ammonia acts as a Lewis base and transfers a lone pair of electrons to form a bond with a hydrogen ion. The species that gains the electron pair is the Lewis acid; for example, the oxygen atom in H3O+ gains a pair of electrons when one of the H—O bonds is broken and the electrons shared in the bond become localized on oxygen. Depending on the context, a Lewis acid may also be described as an oxidizer or an electrophile.
The Brønsted-Lowry definition is the most widely used definition; unless otherwise specified acid-base reactions are assumed to involve the transfer of a proton (H+) from an acid to a base.
The strength of an acid refers to its ability or tendency to lose a proton. A strong acid is one that completely dissociates in water; in other words, one mole of a strong acid HA dissolves in water yielding one mole of H+ and one mole of the conjugate base, A−, and none of the protonated acid HA. In contrast a weak acid only partially dissociates and at equilibrium both the acid and the conjugate base are in solution. Examples of strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). In water each of these essentially ionizes 100%. The stronger an acid is, the more easily it loses a proton, H+. Two key factors that contribute to the ease of deprotonation are the polarity of the H—A bond and the size of atom A, which determines the strength of the H—A bond. Acid strengths are also often discussed in terms of the stability of the conjugate base.
Stronger acids have a larger Ka and a more negative pKa than weaker acids.
( If you know nothing about these Ka , and pKa it really doesen't matter as you will learn them later in the lesson "equilibrium" )
Sulfonic acids, which are organic oxyacids, are a class of strong acids. A common example is toluenesulfonic acid (tosylic acid). Unlike sulfuric acid itself, sulfonic acids can be solids. In fact, polystyrene functionalized into polystyrene sulfonate is a solid strongly acidic plastic that is filterable.
Superacids are acids stronger than 100% sulfuric acid(H2SO4). Examples of superacids are fluoroantimonic acid, magic acid and perchloric acid. Superacids can permanently protonate water to give ionic, crystalline hydronium "salts". They can also quantitatively stabilize carbocations.
In order to lose a proton, it is necessary that the pH of the system rise above the pKa of the protonated acid. The decreased concentration of H+ in that basic solution shifts the equilibrium towards the conjugate base form (the deprotonated form of the acid). In lower-pH (more acidic) solutions, there is a high enough H+ concentration in the solution to cause the acid to remain in its protonated form, or to protonate its conjugate base (the deprotonated form).
( If you know nothing about these Ka , and pKa it really doesen't matter as you will learn them later in the lesson "equilibrium" )
Solutions of weak acids and salts of their conjugate bases form buffer solutions.
( You'll learn a lot about this sub title "weak acid equilibrium" in a later section in order to make you easy to understand refer the tutorials page for navigating )
Acids are used as catalysts in industrial and organic chemistry; for example, sulfuric acid is used in very large quantities in the alkylation process to produce gasoline. Strong acids, such as sulfuric, phosphoric and hydrochloric acids also effect dehydration and condensation reactions. In biochemistry, many enzymes employ acid catalysis.
(you'll learn a lot about catalysis in a later section )
Monoprotic acids are those acids that are able to donate one proton per molecule during the process of dissociation (sometimes called ionization) as shown below (symbolized by HA):
HA(aq) + H2O(l) is in equilibrium with H3O+(aq) + A−(aq) Ka
Common examples of monoprotic acids in mineral acids include hydrochloric acid (HCl) and nitric acid (HNO3). On the other hand, for organic acids the term mainly indicates the presence of one carboxylic acid group and sometimes these acids are known as monocarboxylic acid. Examples in organic acids include formic acid (HCOOH), acetic acid (CH3COOH) and benzoic acid (C6H5COOH).
Polyprotic acids, also known as polybasic acids, are able to donate more than one proton per acid molecule, in contrast to monoprotic acids that only donate one proton per molecule. Specific types of polyprotic acids have more specific names, such as diprotic acid (two potential protons to donate) and triprotic acid (three potential protons to donate).
A diprotic acid (here symbolized by H2A) can undergo one or two dissociations depending on the pH.
H2A(aq) + H2O(l) is in equilibrium with H3O+(aq) + HA−(aq)
HA−(aq) + H2O(l) is in equilibrium with H3O+(aq) + A2−(aq)
A triprotic acid (H3A) can undergo one, two, or three dissociations.
H3A(aq) + H2O(l) is in equilibrium with H3O+(aq) + H2A−(aq)
H2A−(aq) + H2O(l) is in equilibrium with H3O+(aq)+ HA2−(aq)
HA2−(aq) + H2O(l) is in equilibrium with H3O+(aq) + A3−(aq)
An inorganic example of a triprotic acid is orthophosphoric acid (H3PO4), usually just called phosphoric acid. All three protons can be successively lost to yield H2PO4−, then HPO42-, and finally PO43-, the orthophosphate ion, usually just called phosphate.
An organic example of a triprotic acid is citric acid, which can successively lose three protons to finally form the citrate ion. Even though the positions of the protons on the original molecule may be equivalent, the successive Ka values will differ since it is energetically less favorable to lose a proton if the conjugate base is more negatively charged.
Although the subsequent loss of each hydrogen ion is less favorable, all of the conjugate bases are present in solution. For example, a generic diprotic acid will generate 3 species in solution: H2A, HA-, and A2-.
H2A(aq) + H2O(l) is in equilibrium with H3O+(aq) + HA−(aq)
HA−(aq) + H2O(l)is in equilibrium with H3O+(aq) +A2-(aq)
An acid (from the Latin acidus/acēre meaning sour) is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH under 7, with acidity increasing the lower the pH. Chemicals or substances having the property of an acid are said to be acidic.
Common examples of acids include acetic acid ( CH3COOH )(in vinegar), sulfuric acid ( H2SO4(used in car batteries), and tartaric acid/racemic acid( C4H6O6 ) (used in baking). As these three examples show, acids can be solutions, liquids, or solids. Gases such as hydrogen chloride can be acids as well when dissolved in water. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
There are three common definitions for acids:
- the Arrhenius definition,
- the Brønsted-Lowry definition, and
- the Lewis definition.
The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. Most acids encountered in everyday life are aqueous solutions, or can be dissolved in water, and these two definitions are most relevant. The reason why pHs of acids are less than 7 is that the concentration of hydronium ions is greater than 10−7 moles per liter. Since pH is defined as the negative logarithm of the concentration of hydronium ions, acids thus have pHs of less than 7. By the Brønsted-Lowry definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments.
In chemistry, the Lewis definition of acidity is frequently encountered. Lewis acids are electron-pair acceptors. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride. Hydronium ions are acids according to all three definitions. Interestingly, although alcohols and amines can be Brønsted-Lowry acids as mentioned above, they can also function as Lewis bases due to the lone pairs of electrons on their oxygen and nitrogen atoms.
Arrhinius definition
Arrhenius acidsThe Swedish chemist Svante Arrhenius attributed the properties of acidity to hydrogen in 1884. An Arrhenius acid is a substance that increases the concentration of the hydronium ion, H3O+, when dissolved in water. This definition stems from the equilibrium dissociation of water into hydronium and hydroxide (OH−) ions:
H2O(l) + H2O(l) is in equilibrium with H3O+(aq) + OH−(aq)
In pure water the majority of molecules exist as H2O, but a small number of molecules are constantly dissociating and re-associating. Pure water is neutral with respect to acidity or basicity because the concentration of hydroxide ions is always equal to the concentration of hydronium ions. An Arrhenius base is a molecule which increases the concentration of the hydroxide ion when dissolved in water. Note that chemists often write H+(aq) and refer to the hydrogen ion when describing acid-base reactions but the free hydrogen nucleus, a proton, does not exist alone in water, it exists as the hydronium ion, H3O+
Brønsted-Lowry definition
Brønsted-Lowry acidsWhile the Arrhenius concept is useful for describing many reactions, it is also quite limited in its scope. In 1923 chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently recognized that acid-base reactions involve the transfer of a proton. A Brønsted-Lowry acid (or simply Brønsted acid) is a species that donates a proton to a Brønsted-Lowry base. Brønsted-Lowry acid-base theory has several advantages over Arrhenius theory. Consider the following reactions of acetic acid ( CH3COOH ), the organic acid that gives vinegar its characteristic taste
Both theories easily describe the first reaction: CH3COOH acts as an Arrhenius acid because it acts as a source of H3O+ when dissolved in water, and it acts as a Brønsted acid by donating a proton to water. In the second example CH3COOH undergoes the same transformation, in this case donating a proton to ammonia (NH3), but cannot be described using the Arrhenius definition of an acid because the reaction does not produce hydronium. Brønsted-Lowry theory can also be used to describe molecular compounds, whereas Arrhenius acids must be ionic compounds. Hydrogen chloride (HCl) and ammonia combine under several different conditions to form ammonium chloride, NH4Cl. In aqueous solution HCl behaves as hydrochloric acid and exists as hydronium and chloride ions. The following reactions illustrate the limitations of Arrhenius's definition:
H3O+(aq) + Cl−(aq) + NH3 → Cl−(aq) + NH4+(aq)
HCl(benzene) + NH3(benzene) → NH4Cl(s)
HCl(g) + NH3(g) → NH4Cl(s)
As with the acetic acid reactions, both definitions work for the first example, where water is the solvent and hydronium ion is formed. The next two reactions do not involve the formation of ions but are still proton transfer reactions. In the second reaction hydrogen chloride and ammonia (dissolved in benzene) react to form solid ammonium chloride in a benzene solvent and in the third gaseous HCl and NH3 combine to form the solid.
Lewis definition
Lewis acidsA third concept was proposed in 1923 by Gilbert N. Lewis which includes reactions with acid-base characteristics that do not involve a proton transfer. A Lewis acid is a species that accepts a pair of electrons from another species; in other words, it is an electron pair acceptor. Brønsted acid-base reactions are proton transfer reactions while Lewis acid-base reactions are electron pair transfers. All Brønsted acids are also Lewis acids, but not all Lewis acids are Brønsted acids. Contrast the following reactions which could be described in terms of acid-base chemistry.
In the first reaction a fluoride ion, F−, gives up an electron pair to boron trifluoride (BF3to form the product tetrafluoroborate (BF4-. Fluoride "loses" a pair of valence electrons because the electrons shared in the B—F bond are located in the region of space between the two atomic nuclei and are therefore more distant from the fluoride nucleus than they are in the lone fluoride ion. BF3 is a Lewis acid because it accepts the electron pair from fluoride. This reaction cannot be described in terms of Brønsted theory because there is no proton transfer. The second reaction can be described using either theory. A proton is transferred from an unspecified Brønsted acid to ammonia, a Brønsted base; alternatively, ammonia acts as a Lewis base and transfers a lone pair of electrons to form a bond with a hydrogen ion. The species that gains the electron pair is the Lewis acid; for example, the oxygen atom in H3O+ gains a pair of electrons when one of the H—O bonds is broken and the electrons shared in the bond become localized on oxygen. Depending on the context, a Lewis acid may also be described as an oxidizer or an electrophile.
The Brønsted-Lowry definition is the most widely used definition; unless otherwise specified acid-base reactions are assumed to involve the transfer of a proton (H+) from an acid to a base.
Acid strength
The strength of an acid refers to its ability or tendency to lose a proton. A strong acid is one that completely dissociates in water; in other words, one mole of a strong acid HA dissolves in water yielding one mole of H+ and one mole of the conjugate base, A−, and none of the protonated acid HA. In contrast a weak acid only partially dissociates and at equilibrium both the acid and the conjugate base are in solution. Examples of strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). In water each of these essentially ionizes 100%. The stronger an acid is, the more easily it loses a proton, H+. Two key factors that contribute to the ease of deprotonation are the polarity of the H—A bond and the size of atom A, which determines the strength of the H—A bond. Acid strengths are also often discussed in terms of the stability of the conjugate base.
Stronger acids have a larger Ka and a more negative pKa than weaker acids.
( If you know nothing about these Ka , and pKa it really doesen't matter as you will learn them later in the lesson "equilibrium" )
Sulfonic acids, which are organic oxyacids, are a class of strong acids. A common example is toluenesulfonic acid (tosylic acid). Unlike sulfuric acid itself, sulfonic acids can be solids. In fact, polystyrene functionalized into polystyrene sulfonate is a solid strongly acidic plastic that is filterable.
Superacids are acids stronger than 100% sulfuric acid(H2SO4). Examples of superacids are fluoroantimonic acid, magic acid and perchloric acid. Superacids can permanently protonate water to give ionic, crystalline hydronium "salts". They can also quantitatively stabilize carbocations.
weak acid equilibrium
In order to lose a proton, it is necessary that the pH of the system rise above the pKa of the protonated acid. The decreased concentration of H+ in that basic solution shifts the equilibrium towards the conjugate base form (the deprotonated form of the acid). In lower-pH (more acidic) solutions, there is a high enough H+ concentration in the solution to cause the acid to remain in its protonated form, or to protonate its conjugate base (the deprotonated form).
( If you know nothing about these Ka , and pKa it really doesen't matter as you will learn them later in the lesson "equilibrium" )
Solutions of weak acids and salts of their conjugate bases form buffer solutions.
( You'll learn a lot about this sub title "weak acid equilibrium" in a later section in order to make you easy to understand refer the tutorials page for navigating )
Acid catalysis
Acids are used as catalysts in industrial and organic chemistry; for example, sulfuric acid is used in very large quantities in the alkylation process to produce gasoline. Strong acids, such as sulfuric, phosphoric and hydrochloric acids also effect dehydration and condensation reactions. In biochemistry, many enzymes employ acid catalysis.
(you'll learn a lot about catalysis in a later section )
Chemical characteristics
- Monoprotic acids
Monoprotic acids are those acids that are able to donate one proton per molecule during the process of dissociation (sometimes called ionization) as shown below (symbolized by HA):
HA(aq) + H2O(l) is in equilibrium with H3O+(aq) + A−(aq) Ka
Common examples of monoprotic acids in mineral acids include hydrochloric acid (HCl) and nitric acid (HNO3). On the other hand, for organic acids the term mainly indicates the presence of one carboxylic acid group and sometimes these acids are known as monocarboxylic acid. Examples in organic acids include formic acid (HCOOH), acetic acid (CH3COOH) and benzoic acid (C6H5COOH).
- Polyprotic acids
Polyprotic acids, also known as polybasic acids, are able to donate more than one proton per acid molecule, in contrast to monoprotic acids that only donate one proton per molecule. Specific types of polyprotic acids have more specific names, such as diprotic acid (two potential protons to donate) and triprotic acid (three potential protons to donate).
A diprotic acid (here symbolized by H2A) can undergo one or two dissociations depending on the pH.
H2A(aq) + H2O(l) is in equilibrium with H3O+(aq) + HA−(aq)
HA−(aq) + H2O(l) is in equilibrium with H3O+(aq) + A2−(aq)
A triprotic acid (H3A) can undergo one, two, or three dissociations.
H3A(aq) + H2O(l) is in equilibrium with H3O+(aq) + H2A−(aq)
H2A−(aq) + H2O(l) is in equilibrium with H3O+(aq)+ HA2−(aq)
HA2−(aq) + H2O(l) is in equilibrium with H3O+(aq) + A3−(aq)
An inorganic example of a triprotic acid is orthophosphoric acid (H3PO4), usually just called phosphoric acid. All three protons can be successively lost to yield H2PO4−, then HPO42-, and finally PO43-, the orthophosphate ion, usually just called phosphate.
An organic example of a triprotic acid is citric acid, which can successively lose three protons to finally form the citrate ion. Even though the positions of the protons on the original molecule may be equivalent, the successive Ka values will differ since it is energetically less favorable to lose a proton if the conjugate base is more negatively charged.
Although the subsequent loss of each hydrogen ion is less favorable, all of the conjugate bases are present in solution. For example, a generic diprotic acid will generate 3 species in solution: H2A, HA-, and A2-.
H2A(aq) + H2O(l) is in equilibrium with H3O+(aq) + HA−(aq)
HA−(aq) + H2O(l)is in equilibrium with H3O+(aq) +A2-(aq)